Registrar Resource Center
Everything you need to know about Certification or Accreditation
Free Quotes from
Certification & Accreditation Bodies
Looking for the right Certification Body (also called Registrar) or Accreditation Body to audit your company can be confusing. That is why we have partnered with many different accredited Certification and Accreditation Bodies to make it easy for you to find, choose, and hire one to complete your company’s needs.
- Click the red ‘Get Started’ Button
- Select Your Country
- Choose Your Standard
- Select 3 Accredited CBs / ABs to Receive Quotes From
- Complete the Form with your Organization’s Info
- Submit!
Then What?
- The certification form is automatically sent to the Certification Bodies and/or Accreditation Bodies you chose.
- You will receive a copy of your submittal, and we retain one for our records. (We never sell your information to third parties.)
- You will receive a quotation within 72 hours so you may select the option that will best fit your unique requirements.
ISO Certification & Accreditation Basics
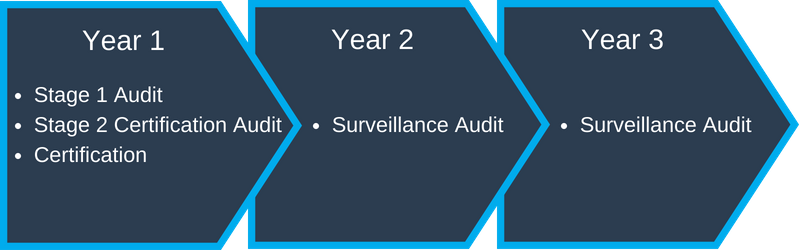
Generally, when you sign a contract with a Certification or Accreditation Body, it will include the Certification or Accreditation Audit, and Surveillance Audits. The Certification or Accreditation Audit is the initial audit that will be done to see if you will achieve your desired certification, whether it is ISO 9001, IATF 16949, or ISO/IEC 17025, for example. Once you are approved, your certificate will be valid for three years (See typical Certification or Accreditation Process below).
After you have your certification or accreditation, the Certification or Accreditation Body will come back every 6 months or year for a Surveillance Audit to see if you are maintaining your system and continuing to meet the requirements of the standard. Certification and Accreditation Bodies vary in their approach, so you will want to find out details from each of them you are interested in.
Your certificate will be valid for 3 years. After that period you will be required to do another Certification or Accreditation Audit to renew your certificate.
When to Select a Certification or Accreditation Body
Organizations often wait until they feel their QMS is running smoothly before they select a Certification or Accreditation Body. However, we recommend choosing them earlier in the process. Here’s why:
- This assures that you can find one who is able to meet your timeframe.
- The advantage of interviewing them early is that they will ultimately be the ones who will evaluate your QMS. A Certification or Accreditation Body CANNOT consult for a company who they audit, but they can explain (based on their experience) how they intend to audit your organization. So, if you choose them earlier in the process you can ask them questions along the way. It’s like asking the teacher what you’re going to be tested on. This can be important because much is up to the individual’s discretion (like a referee in a sporting event) and you would be wise to consider it.
ISO Certification or Accreditation Process
ISO standards for a Quality Management Sytem (“QMS”) require each organization complete internal audits for their QMS to confirm the processes are being managed correctly. In other words, internal audits confirm the organization is fully in control of its activities and all requirements of the QMS standard, along with customer and statutory and regulatory requirements.
To achieve certification, an organization must hire an independent Certification or Accreditation body (sometimes known as a registrar) to perform an external audit, and obtain an ISO certificate of conformity.
Click each part for more details on the Certification or Accreditation process!
Enquiry
Complete Questionnaire
Each Certification Body (also called a “Registrar”) or Accreditation Body may have questions about your application.
We’ve provided a free Registrar Questionnaire Checklist to help you interview your registrars.
Proposal
Review the proposal to ensure it meets your requirements. Remember, YOU are the customer in this situation and have a choice in which Certification or Accreditation Body you choose – this is not like a government inspector where you only have one choice. You should ensure that they meet all of your requirements:
- Technical Requirements – Do they understand your business?
- Commercial Requirements – Can they meet your desired timeline?
Confirm Application and Schedule

You will enter into a three (3) year contract with the Certification or Accreditation Body, which outlines obligations, liability, confidentiality and access rights. The schedule will be as follows:
- Year 1 – Complete Certification or Accreditation Audit
- Year 2 & 3 – Surveillance Audits – usually does not include documentation review
You will start over with a complete Audit in year 4 with the same, or another Certification or Accreditation Body – it is your choice.
1st Stage Assessment
After your quality system has been implemented, the Certification or Accreditation Body conducts a Stage 1 Audit to assess your documentation and verify key practices are in place. This includes internal audits, management reviews, and tracking performance. If you successfully pass this audit without any major issues, the Certification or Accreditation Body will confirm your readiness for the full audit. (Learn more about the 2-Stage Regisration Audit.)
The Initial Certification or Accreditation Audit
The assessment process for achieving certification or accreditation consists of a two stage Initial Certification or Accreditation Audit:
Stage 1 – The purpose of this visit is to confirm the readiness of the organization for a full assessment. The auditor will:
- Check that the documented Information conforms to the requirements of ISO standard
- Confirm its implementation status
- Confirm the scope of certification or accreditation
- Review legislative compliance
- Generate a report that identifies any non-compliance or possibilities for non-compliance and agree to a corrective action plan if required.
- Generate an assessment plan and confirm a date for the Stage 2 Audit in your company
During the Stage 1 Audit, only a few employees will be interviewed. If there are no significant problems, the Stage 2 Audit will normally proceed in one or two months.
Certification or Accreditation Assessment (Stage 2 Audit)
One or two months after an effective Stage 1 Audit, the certification or accreditation body will return to audit the entire system. They will look for conformity to customer, legal, and executive requirements, as well as, to the other requirements of the ISO standard.
The audit duration will depend on the size of the organization, the number of sites, and the complexity of the processes included in the system. The number of days for the audit is based on ISO 17021. (See how to calculate number of audit days for your organization). For example, a small company with 10 or fewer employees might get an audit of only two days. For a company of 20 employees, the duration would rise to three days.
If the organization receives no major nonconformities during the Stage 2 Audit, the audit team will recommend certification based on your compliance with an acceptable corrective action plan for any stated minor nonconformities.
If one or more major nonconformities are found, the certification or accreditation body will either conduct a special visit in a month or two to confirm the major issues have been resolved by use of the PDCA method, or they will conduct another full certification audit when the organization says the major nonconformities have been corrected.
Stage 2 – The purpose of this visit is to confirm that the quality management system fully conforms to the requirements of the ISO standard in practice. The auditor will:
- Complete sample audits of the processes and activities defined in the scope of assessment
- Record how the system complies with the standard
- Record how the system complies with the organizations’ documented information
- Report any non-conformances or potential for non-conformance
- Produce a surveillance plan and confirm a date for the first surveillance visit
During the Stage 2 Audit, several employees will be interviewed. If the auditor identifies any major non-conformance, the organization cannot be certified until corrective action is taken and verified. This means the auditor has to come back out for an on-site verification visit.
If there are no major non-conformances, then the certificate is typically sent out within 30-45 days after the Stage 2 Audit.
Major Non-Conformance
After the Certification or Accreditation Assessment is complete, it will reveal whether or not there are any non-conformances with the standard.
Corrective Action
Corrective Action if Necessary – Your organization will need to continually analyze, document, and correct non-conformances for as long as you have a QMS.
Follow Up
Depending on the size of the organization, the certification or accreditation body will establish an annual or semi-annual surveillance program. The total surveillance days each year will be about one-third the duration of the Stage 2 Audit. Each visit will always assess certain key elements of the system, for example, internal audit, management review, customer satisfaction, and corrective action. A sample of the other areas of the system will be examined during the visits, with all the areas being assessed over the three-year life of the certificate.
Recertification or Re-Accreditation Audit
Every three years, the entire system will be assessed again. The recertification audit duration will be about two-thirds as long as the Stage 2 Audit. Assuming the assessment doesn’t find any major non-conformances, the audit team can recommend the organization for continued certification or accreditation. And, after receipt of an acceptable corrective plan for any minor non-conformances, the certification or accreditation body will reissue the ISO certificate.
Costs
The costs for the audits and registration will be dependant on the size of your company, the number of locations, the accreditations that you need, and the distance between you and the auditor assigned by your Registrar.
The costs are typically dependant on the number of audit days required for the registration audit and the surveillance audits, the travel costs for the auditors, and the administration fees and accreditation fees for the registration.
Choosing a Registrar
When choosing a certification or Accreditation body for ISO certification, these are the aspects the organization needs to take into account.
- An independent QMS audit to confirm ISO certification is a business decision:
- Does a customer or agency require it?
- Does it help with risk mitigation?
- Would it improve public relations?
- Cost is not the only consideration. Criteria to consider include:
- Evaluate several certification bodies using our Registrar Checklist.
- Has the certification or accreditation body been accredited? Accreditation, in simple terms, means that a certification body has been approved to certify organizations.
- Is their accreditation Internationally recognized/accepted? There are some organizations who are not internationally recognized.
- Does the Certification Body follow ISO/IEC 17021:2015? ISO 17021 Conformity assessment – Requirements for bodies providing audit and certification of management systems?
- Do the auditors have experience in your industry?
- Is the Certification or Accreditation Body approved for certifications or accreditations you may consider in the future?
The most important factor in choosing a Registrar is how well they can work with you. This includes how well they know your industry, how much experience they have with similar companies, and how well they communicate with you and your employees. There are many rules that a registrar must follow, issued by organizations like the ANAB. See ANAB Rules…
Interview 3 or more Registrars to get a good idea of the options available and differences between Registrars. Look locally if you have good choices, it will save on costs, but if you do not find a good fit look farther. The benefits of your relationship with your Registrar will pay off. Remember that these are experienced professionals that spend day after day evaluating how companies do business. The feedback you get from them is one of the best ISO Certification or Accreditation benchmarking tools available.


